

AMBROXOL (CAS NO : 135048-43-0)
Ambroxol Hydrochloride is a mucolytic agent that facilitates the clearance of mucus from the respiratory tract. It is commonly used in the treatment of respiratory diseases such as bronchitis, asthma, and chronic obstructive pulmonary disease (COPD). Ambroxol works by breaking down the structure of mucus, making it easier to expel. AMBROXOL is a small molecule drug with a maximum clinical trial phase of III (across all indications) and has 7 investigational indications. Other identifiers such UNII 200168S0CL and QH6ZT6J071.

Ambroxol Hydrochloride is a mucolytic agent that facilitates the clearance of mucus from the respiratory tract. It is commonly used in the treatment of respiratory diseases such as bronchitis, asthma, and chronic obstructive pulmonary disease (COPD). Ambroxol works by breaking down the structure of mucus, making it easier to expel. AMBROXOL is a small molecule drug with a maximum clinical trial phase of III (across all indications) and has 7 investigational indications. Other identifiers such UNII 200168S0CL and QH6ZT6J071.

.3d8f8f41.svg)
Industries
Pharmaceutical
.3556d45a.svg)

Category
Pharmaceutical Actives & Precursors


Sub-category
Intermediates & Precursors
Get a Quote
Details included in quote
Minimum Order Quantity
Lead Time
Regional Availability
Incoterms
.7767eb0f.png)

Chemical Properties & Specifications
Applications of AMBROXOL
Pharmaceuticals
Used as an active ingredient in formulations for treating respiratory conditions by reducing mucus viscosity.
Cough Syrups and Tablets
Incorporated into over-the-counter and prescription medications aimed at alleviating productive coughs.
Have Questions About AMBROXOL?
We've Got Answers.
Our Top Specialty Chemical Products in USA
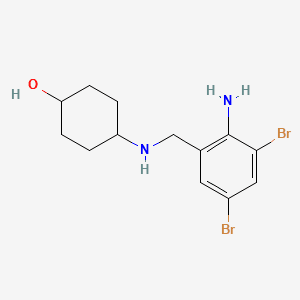
Antimony Trioxide
CAS No. : 1309-64-4
Category : Synergists & Smoke Suppressants
Sub-Category : Antimony-based synergists
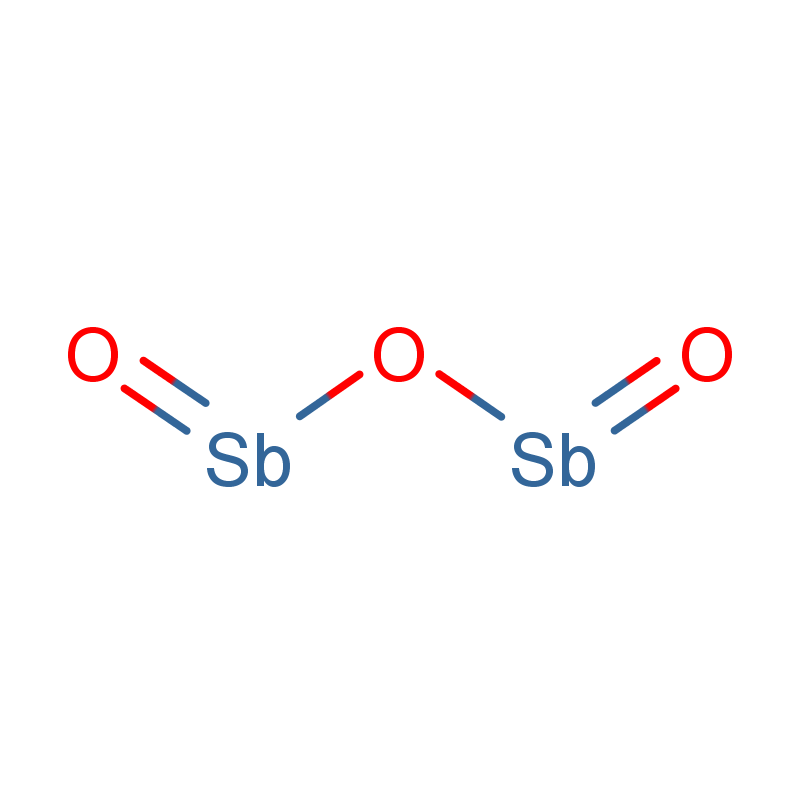
Ferric Chloride Liquid
CAS No. : 7705-08-0
Category : Inorganic Chemicals
Sub-Category : Metal-Based Coagulants
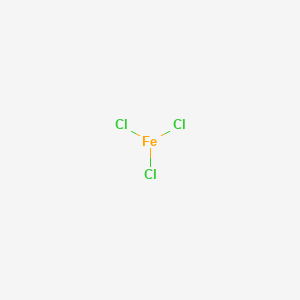
Hydrogen Peroxide 50%
CAS No. : 7722-84-1
Category : Inorganic Chemicals
Sub-Category : Peroxides & Oxidizing Agents

Titanium Dioxide ATR-312
CAS No. : 13463-67-7
Category : Pigments & Colorants
Sub-Category : Inorganic Pigments

Titanium Dioxide Rutile R6618(T)
CAS No. : 13463-67-7
Category : Pigments & Colorants
Sub-Category : Inorganic Pigments
Dibutyl Ether
CAS No. : 142-96-1
Category : Solvents & Carriers
Sub-Category : Ethers & Ether-Based Solvents